Interventional Cardiology Section
| Publications |
| Last updated: 05-10-21 |
1.Cardiac Catheterization Laboratory Volume Changes During COVID-19—Findings from a Cardiovascular Fellows Consortium https://www.ajconline.org/article/S0002-9149(20)30565-8/fulltext Kadavath S, Mohan J, Ashraf S, Kassier A, Hawwass D, Madan N, Salehi N, Bernardo M, Mawri S, Rehman KA, Ya'qoub L, Strobel A, Dixon SR, Siraj A, Messenger J, Spears JR, Lopez-Candales A, Madder R, Bailey SR, Alaswad K, Kim MC, Safian RD, Alraies MC.2020 Sep 1;130:168-169 2.How The COVID-19 Pandemic Has Affected Cardiology Fellow Training https://www.ajconline.org/article/S0002-9149(21)00322-2/fulltext Kadavath S MD , Hawwas D MD , Strobel A MD , Mohan J DO , Bernardo M MD , Kassier A MD ,Ya’qoub L MD , Madan N MD MPH , Ashraf S MD , Salehi N MD , Mawri S MD MS , Abdur Rehman K MD , Siraj A MD , Alraies C MD MPH , Saad M MD PhD , Aronow H MD doi: https://doi.org/10.1016/j.amjcard.2021.03.052 |
| Contributors |
| Last updated: 05-10-21 |
Section Co-Leads:
Section Contributors:
|
| Cardiac Catheterization Laboratory |
| Last updated: 05-10-21 |
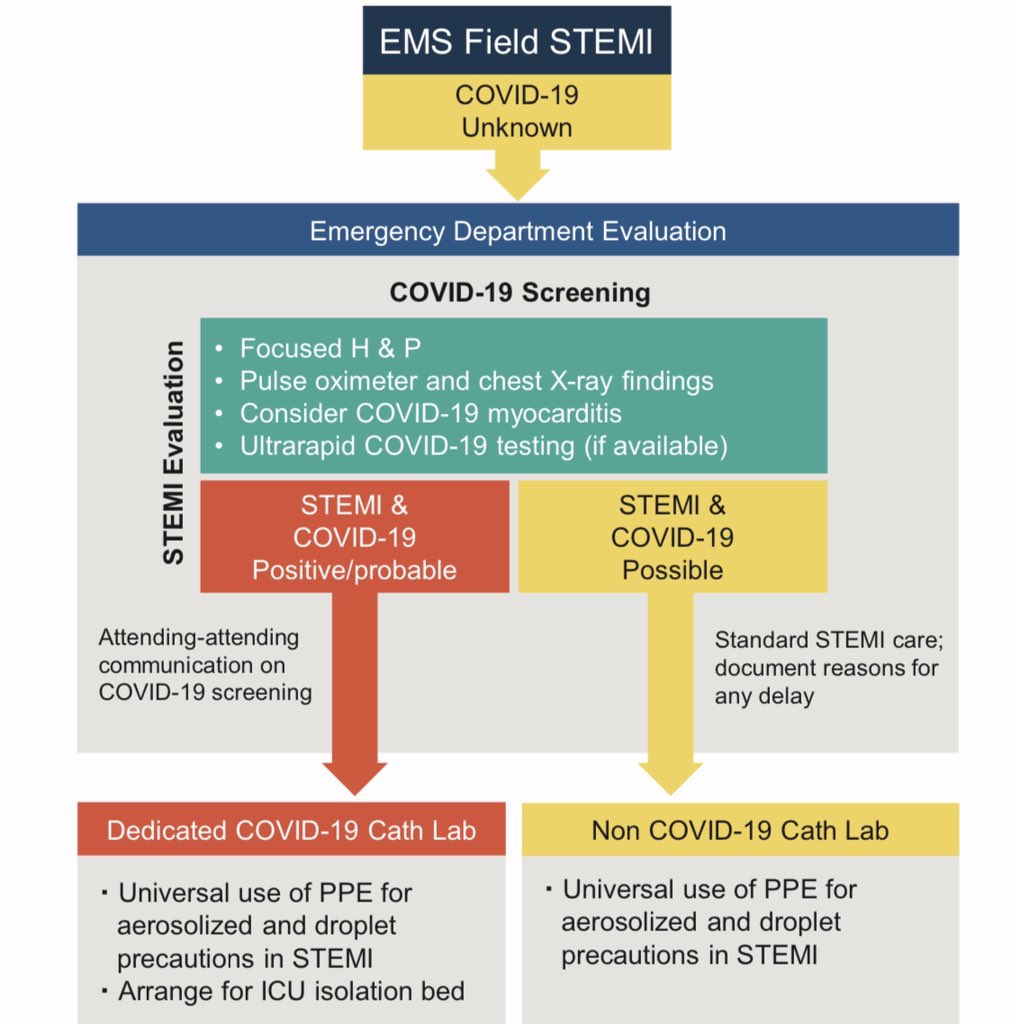
Cardiac Catheterization Laboratory (Welt et al,JACC, 2020)
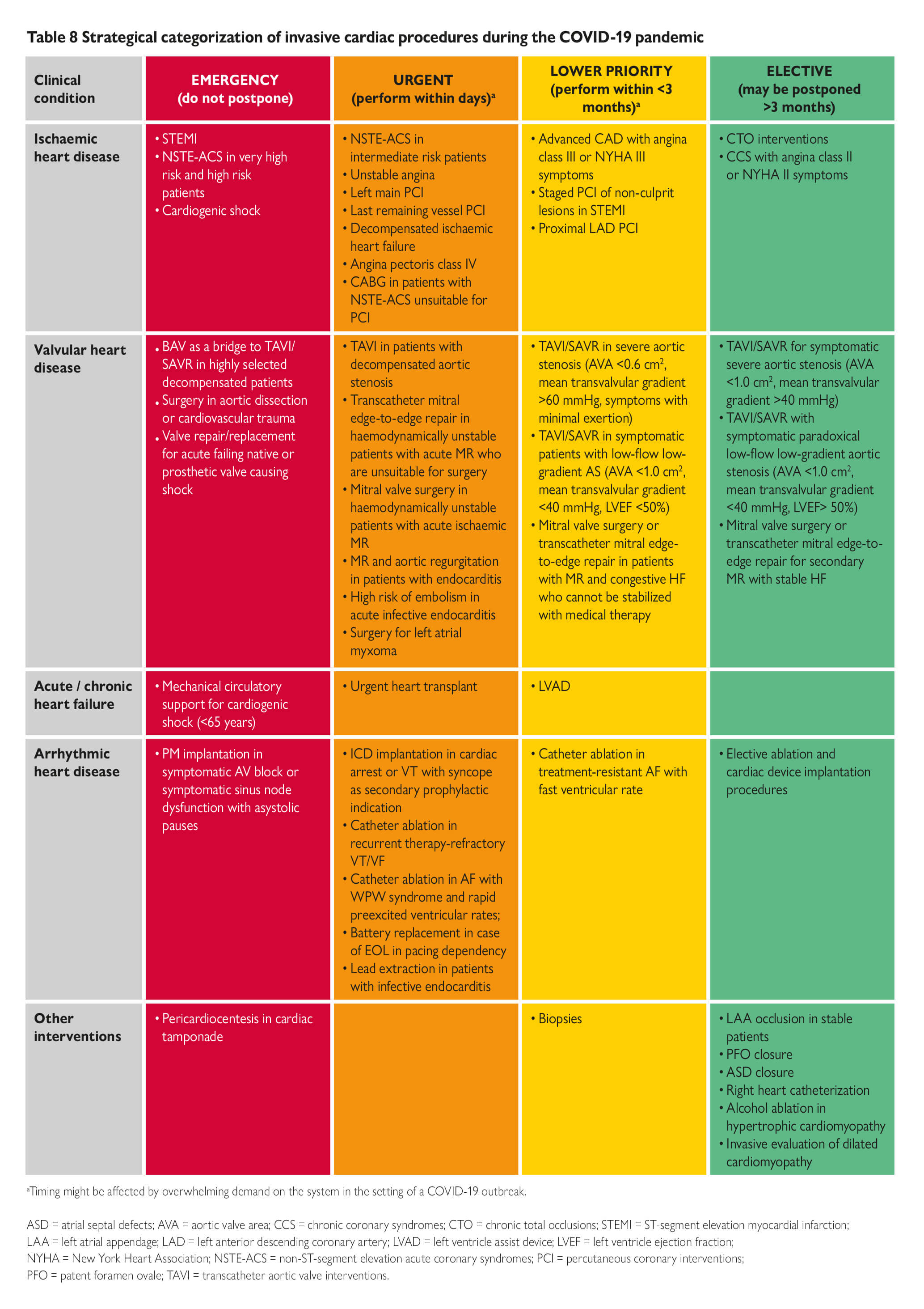
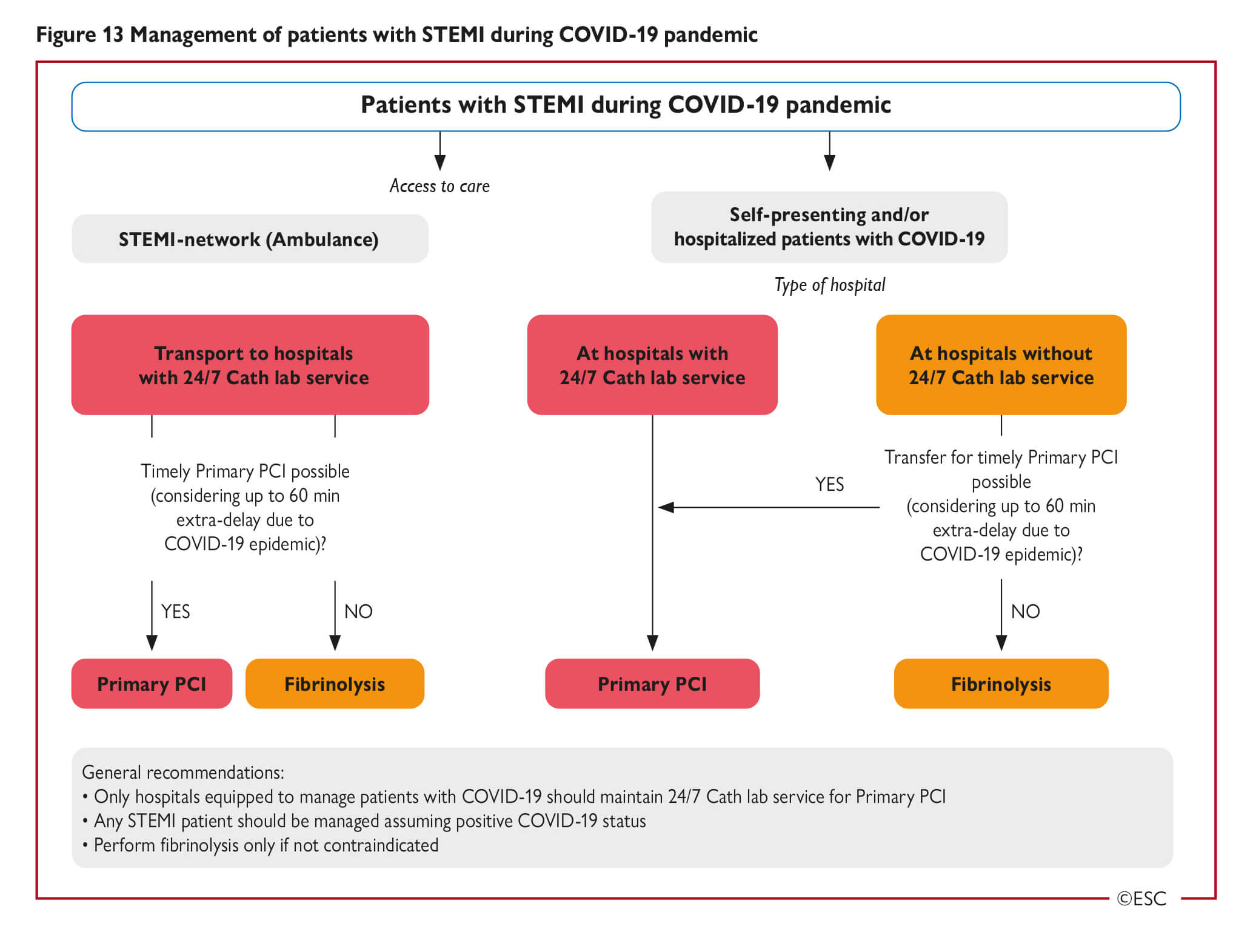
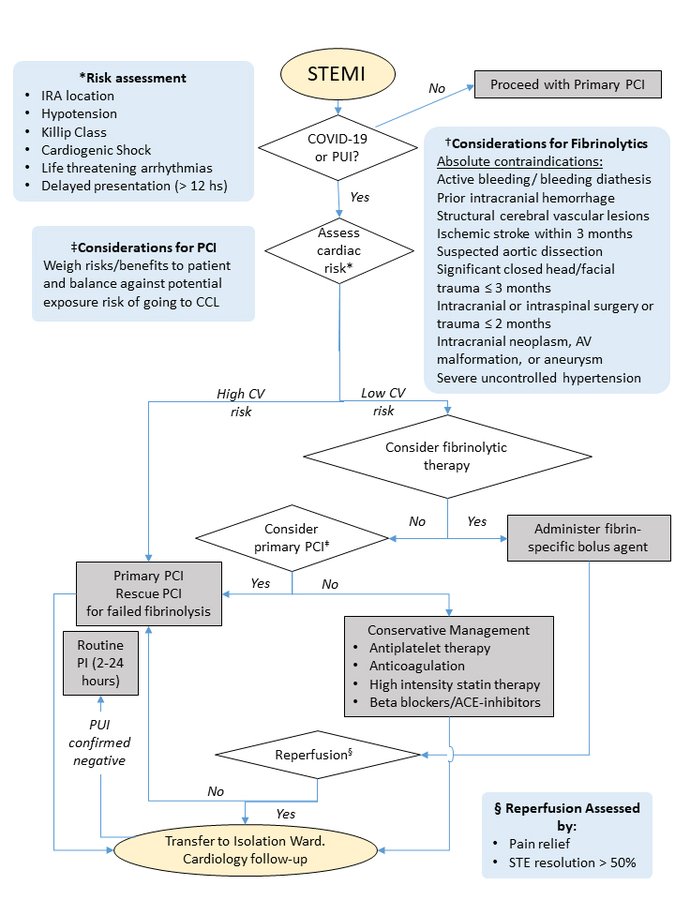
How to balance acute myocardial infarction and COVID-19: the protocols from Sichuan Provincial People’s Hospital (Zeng et al, Intensive Care Medicine, 2020) · Perform rapid nucleic acid test for SARS-CoV-2 if available. · If positive or time for results significantly delays time to reperfusion, follow protocol below Protocol for STEMI: 1. Stable patients: · Onset of symptoms <12 hrs: Perform thrombolytic therapy if no contraindication. If contraindication present, consider infection risk vs. benefits of PCI. After treated and recovered (test negative twice for COVID-19), elective PCI should be considered for those who received thrombolysis. · Onset of symptoms >12 hrs: Consider infection risk vs. benefits of PCI if there is continuing evidence of ischemia. 2. Unstable patients: · Severe pneumonia: Conservative management. · Mild to moderate pneumonia: Assess time of onset and follow STEMI protocol for stable patient Protocol for NSTEMI: · Exclude SARS-CoV-2 infection first. · Conservative medical management until recovery from COVID-19 pneumonia, then reassess the benefit of PCI. · If unstable or malignant arrhythmia, perform PCI in isolated catheter room |
| Last updated: 05-10-21 |
Catheterization Laboratory Considerations During the Coronavirus (COVID-19) Pandemic: From ACC’s Interventional Council and SCAI (Welt et al, JACC, 2020) Key Points as summarized by Ajay Kirtane MD ( @ajaykirtane) |
| Societal Resources |
| Last updated: 05-10-21 |
Considerations for Cardiac Catheterization Laboratory Procedures During the COVID-19 Pandemic (Szerlip et al, CCI, 2020)
Management of Acute Myocardial Infarction During the COVID-19 Pandemic: A Consensus Statement from the Society for Cardiovascular Angiography and Interventions (SCAI), American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP) (Mahmud et al, CCI, 2020)
|
| ESC Recommendations |
| Last updated: 05-10-21 |
European Society of Cardiology (ESC) COVID-19 Guidance Key Points:
Reproduced from the original work here . Permission obtained from © The European Society of Cardiology 2020. All rights reserved. Chronic Coronary Syndrome (ESC Guidance 3/2020)
Key messages:
|
| Acute Coronary Syndrome |
| Last updated: 05-10-21 |
Incidence:
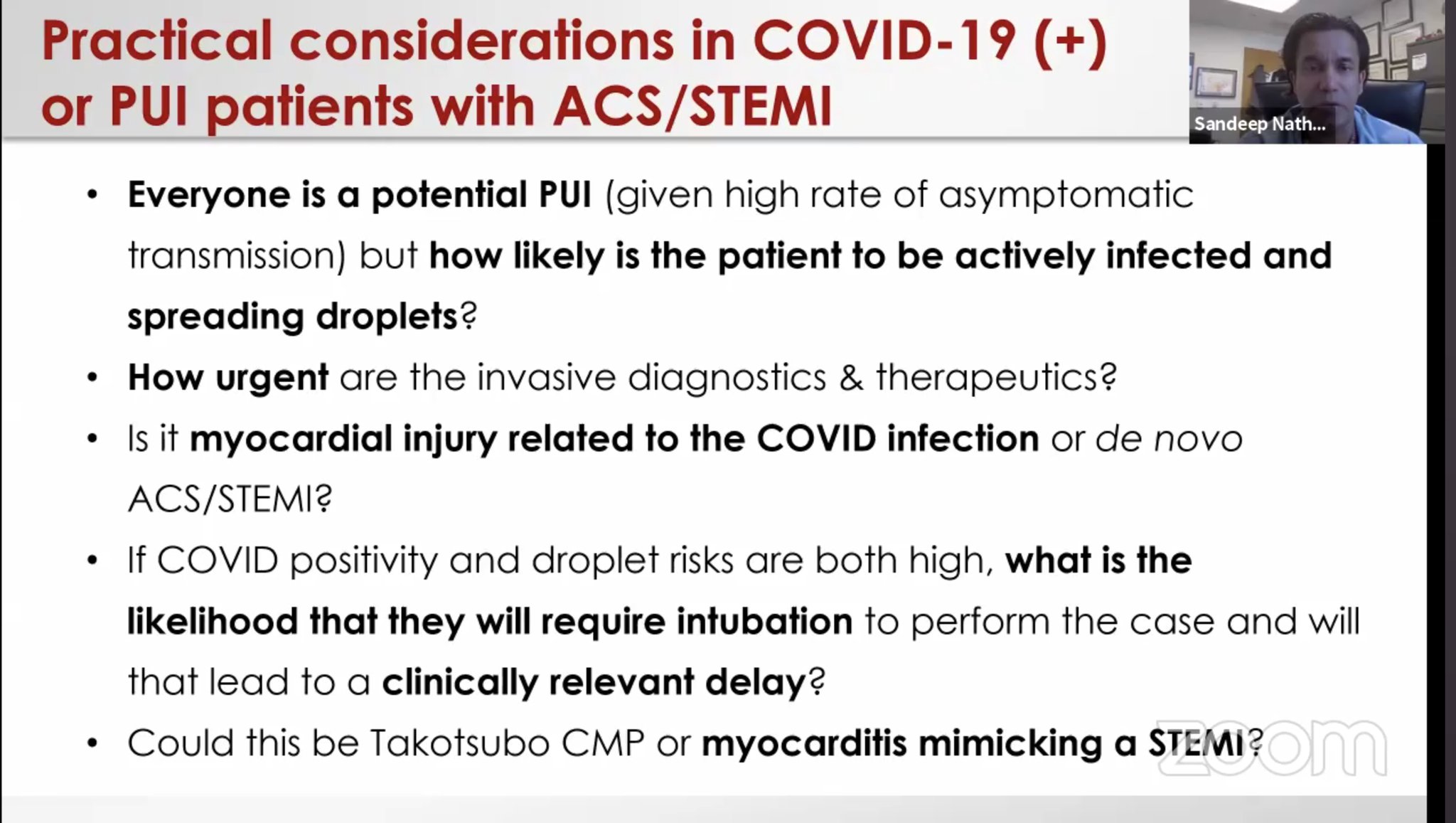
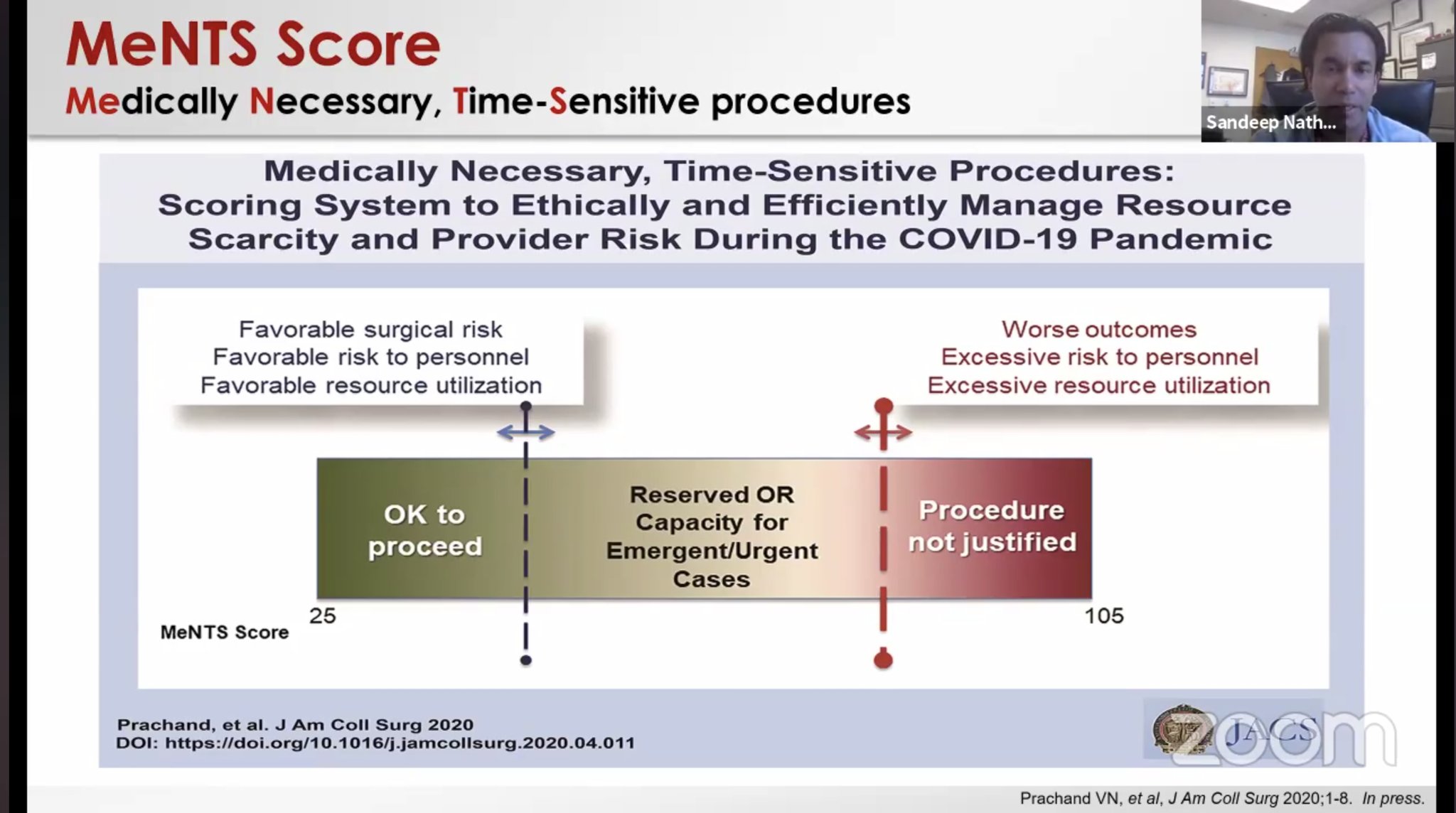
64-yo F, PMH HTN, HLD, came with chest pain and was found to have anterolateral STEMI V2-6, I, AVL, ST depression in AVR, troponin 7.9 ng/ml on admission, LHC showed non-obstructive CAD. RHC showed RAP 10 mmHg, pulmonary artery pressure of 30/20 mmHg, PCWP 21 mmHg and a Fick cardiac index of 1.0 L/min/m2. IABP and Dobutamine was started. Echo EF 30% with small LV associated with thick LV walls and dilated hypokinetic RV. Lactate of 5. She was started on oral hydroxychloroquine 600 mg every 12 hours for 1 day followed by 400 mg daily for 4 additional days. Lactate normalized, troponin peaked at 18.6 then normalized. IABP and inotropes weaned off after 7 days. Hospital day 10, repeat echo EF 50% and reduced LV thickness. Thoughts 1- For hemodynamics: To reduce the potential contamination associated with patient transport to the catheterization suite, bedside placement of a pulmonary artery catheter and IABP may be considered? Other potential indicators of the hemodynamic status may include sampling the central venous saturation or hemodynamic assessment via echocardiography. 2- Does the increased wall thickness present in this patient suggest a characteristic finding of myocarditis-like presentations with COVID-19? 3- A low threshold to assess for shock in acute systolic HF associated with COVID. Similarly, there should be a low threshold for SARS-CoV-2 testing in patients presenting with signs of myopericarditis even in the absence of fever and respiratory symptoms.
38-yo F, DM II, presented with cough, dyspnea and pleuritic chest pain. TTE normal LVEF. CXR pulmonary infiltrates, COVID positive. Respiratory status deteriorated requiring intubation, then paralytic and prone position without significant improvement, PO2 56, pH 7.26, pCO2 40 mmHg. VV ECMO was initiated, L femoral and L IJ. She became hypotensive requiring inotropic support, decrease urine output and lactate of 5. Repeat TTE showed LVEDD of 4.5 cm, LVEF 20-25%, with akinesis of the mid LV segments, and normal RV. High sensitivity troponin T was 1341 ng/L. After a multidisciplinary discussion, a 15F arterial limb was added via the right femoral artery to convert the circuit to veno-arterial-venous (VAV) ECMO, achieving 2L of arterial flow. Over 24 hours, lactate normalized and she was off pressors. ECMO decannulated after 7 days, she remains intubated. Thoughts 1- The cardiac involvement became evident after the initiation of VV ECMO. 2- Direct cardiac injury may occur as the result of viral invasion, or cytokine storm induced by COVID-19 3- Reports of ECMO use in COVID-19 patients are limited. Utility is likely limited due to the extensive resources required to provide the therapy and limited availability. 4- Are such relatively low levels of hemodynamic support sufficient in other cases with COVID-19 related cardiogenic shock? decreased likelihood of LV distention with its associated consequences?
64 yo F, NICM (recovered EF), PAF, HTN, DM, presented with a non-productive cough and shortness of breath for two days. ECG PAC, PVC, prolonged QTc 528 ms. No hydroxychloroquine, given antibiotics for pneumonia, respiratory deterioration requiring intubation, Echo severely reduced EF, RHC with PAP 40/20, Fick’s CI 1.7, hypotension requiring dobutamine (caused NSVT so was discontinued), IABP considered but BP and lactate improved, troponin stable peak 214 ng/ml, remains intubated on day 9, multiple extubation attempts.
Thoughts 1- Neither myocarditis nor cytokine storm were probable mediators of the recurrence of her depressed cardiac function, given the relatively low biomarker levels. 2- Early intubation and reversal of her respiratory failure led to improvements in hemodynamics in the absence of direct cardiac support. 3- RHC to assess hemodynamics 4- Prolonged cQT Case
51 yo M, heart transplantation 2007, renal transplantation 2010, presented with intermittent fever, dry cough, and shortness of breath for 9 days, on immunosuppression: tacrolimus 5 mg BID, mycophenolate mofetil 250 mg BID, and prednisone 5 mg daily. Mx: Mycophenolate mofetil was discontinued, started on hydroxychloroquine and azithromycin as well as ceftriaxone for empiric treatment of pneumonia. Discharged on day 7. Thoughts 1- stopped the mycophenolate mofetil during the infection, with a plan to resume it following full recovery. 2- Programs must balance the risks of new transplant recipients contracting COVID-19 during their hospitalization with the risks of waitlist mortality if transplantation is postponed. 3- COVID-19 may also impact the donor pool due to diversion of crucial resources and fear of disease transmission from donors
(pre COVID19 Guidelines)
Highlights from the Webinar by C3 Academy on The North American Experience in managing patients with ACS/STEMI
Population Trends of interventions for ACS worldwide: In the third most populous region of Italy, a decline of 32% in the number of PCI for ACS was observed (Piccolo et al, Circulation, 2020)
Ventricular septal defect observed in three cases (Moroni et al, JACC CR, 2020)
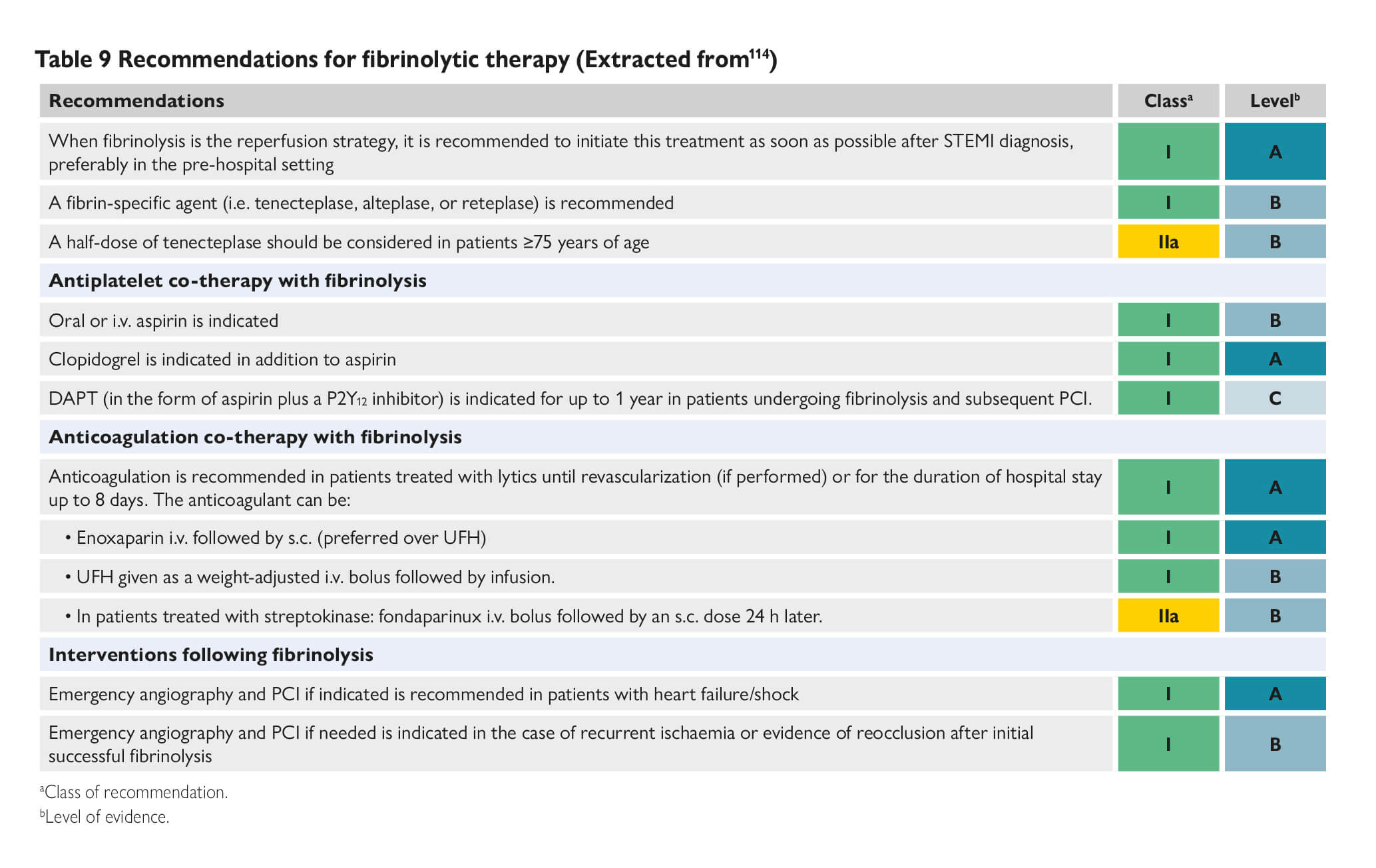
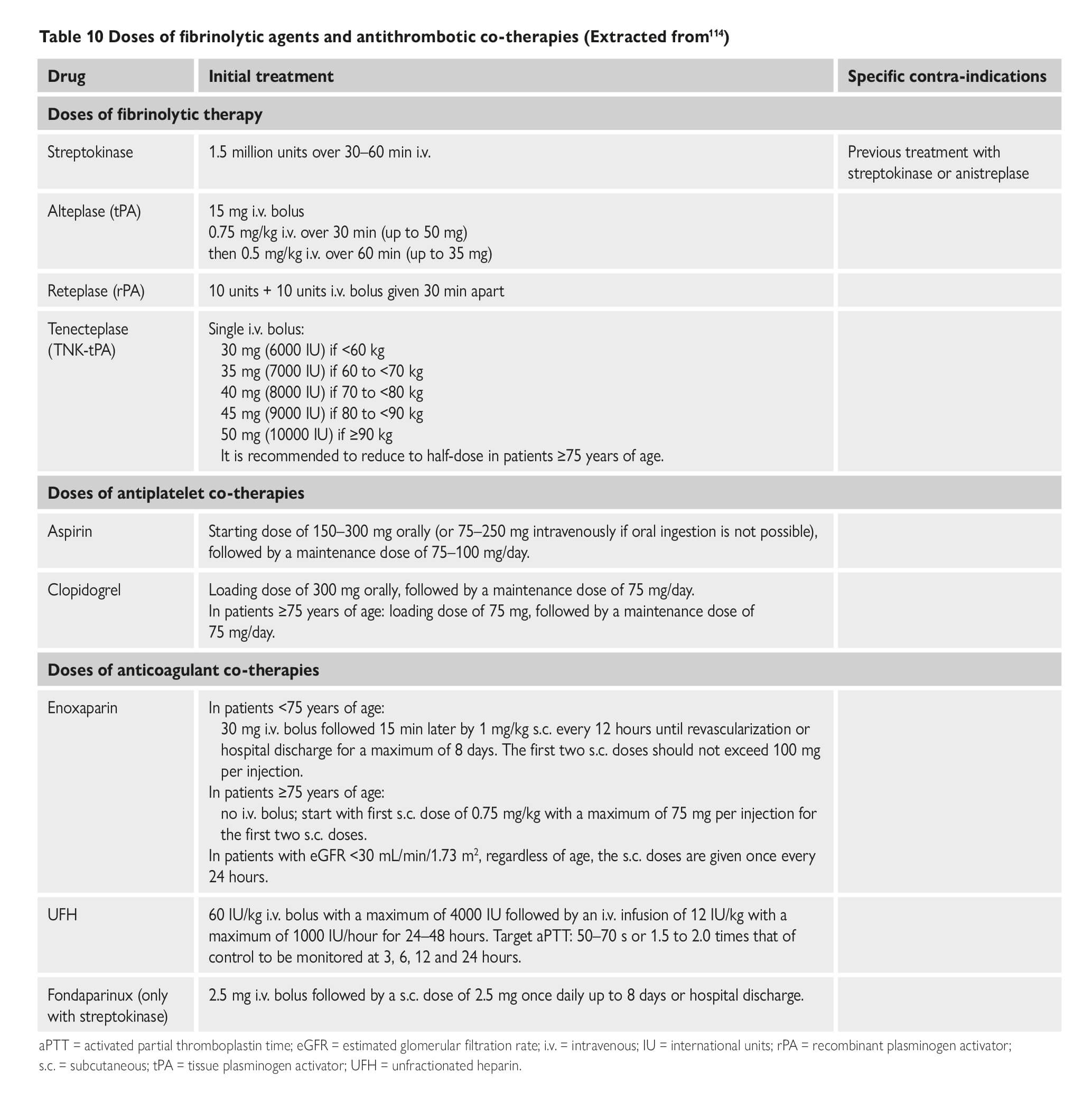
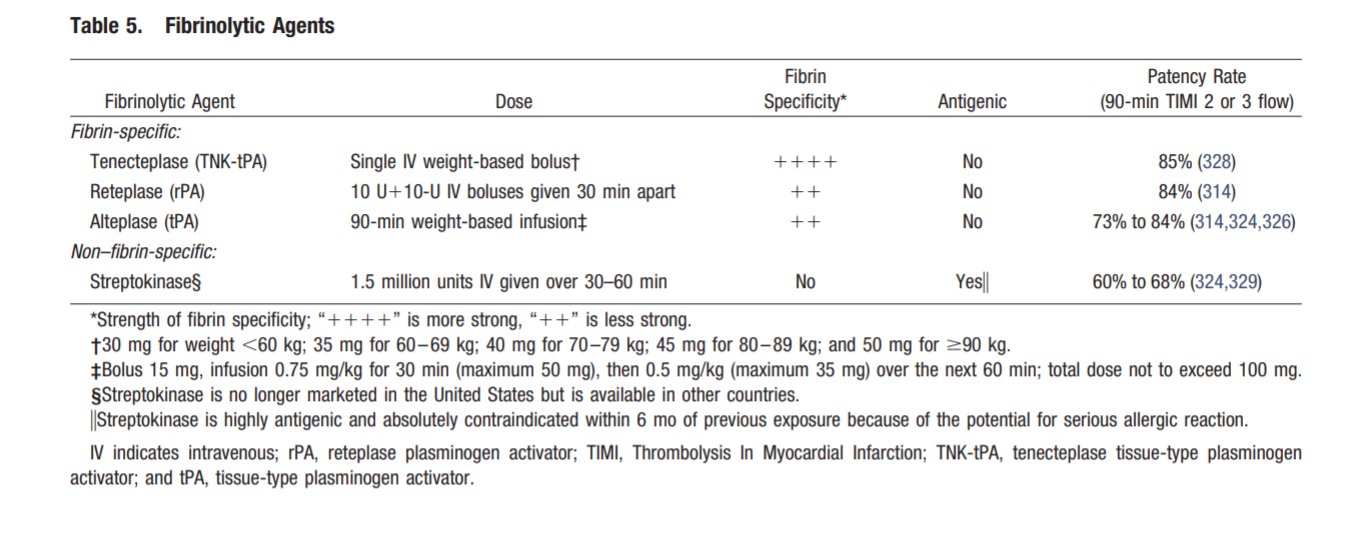
COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up. (Bikdeli et al ) COVID-19 and antithrombotic therapy for acute coronary syndrome (ACS) In presentations consistent with ACS due to plaque rupture (i.e. Type I MI), dual antiplatelet therapy (DAPT) and full dose anticoagulation per American College of Cardiology (ACC)/American Heart Association (AHA) and the European Society of Cardiology (ESC) guidelines should be administered unless there are contraindications. In patients with perceived elevated bleeding risk, regimens with less potent antiplatelet agents, such as with clopidogrel, should be considered. COVID-19 and interventional therapies for ACS. The ACC and Society for Cardiovascular Angiography and Interventions (SCAI) recommendations note that it is reasonable to continue optimal medical therapy and defer non-urgent cardiac procedures, in order to preserve personal protective equipment (PPE), hospital resources, and minimize exposure for patients and healthcare workers. Prior to intervention, efforts should be made to distinguish non-specific myocardial injury, myocarditis, and true plaque rupture presentations. Even in case of STEMI, in which primary percutaneous coronary intervention (PCI) reduces mortality and reinfarction, risk of COVID-19 transmission from patients to healthcare workers must be considered. Disseminated intravascular coagulopathy (DIC) and Considerations for Antithrombotic Therapy Long-acting antiplatelet agents should be discontinued in most patients with DIC, unless required (e.g. recent ACS or stent implantation). In general, it is reasonable to continue dual antiplatelet therapy if platelet count ≥50,000, reduce to single antiplatelet therapy if 25,000≤platelet count. However, these guidelines may be revised upward or downward depending on the individualized relative risk of stent related thrombotic complications vs. bleeding Covid-19 investigational therapies and their interaction with anti-platelet agents Bevacizumab This is a monoclonal antibody that binds to vascular endothelial growth factor (VEGF), and is under investigational use for COVID-19, is associated with increased risk for adverse cardiovascular events, including MI, cerebrovascular accidents, and VTE. Fingolimod It is an immunomodulating agent being tried for COVID-19, may reduce reperfusion injury and improve outcomes in patients suffering from acute ischemic stroke. Hydroxychloroquine It may potentially exert antithrombotic properties, especially against anti-phospholipid antibodies. Lopinavir/ritonavir It is a protease inhibitor and inhibits CYP3A4 metabolism. Although the active metabolite for clopidogrel is mostly formed by CYP2C19, inhibition of CYP3A4 may also lead to reduction in effective dosage of clopidogrel. This inhibition of CYP3A4 may increase effects of ticagrelor. Remdesivir This is a nucleotide-analog inhibitor of RNA-dependent RNA polymerase, reportedly an inducer of CYP3A4; however, dose adjustments for oral antiplatelet agents are currently not recommended Of note, there are no major drug-drug interactions between investigational COVID-19 therapies and parenteral antiplatelet agents such as cangrelor and glycoprotein IIb/IIIa inhibitors. Covid-19 investigational therapies and their interaction with anti-coagulation agents Lopinavir/ritonavir It has the potential to also affect choice and dosage of a number of anticoagulants. For example, vitamin K antagonists, apixaban, and betrixaban may all require dose adjustment, while edoxaban and rivaroxaban should not be 13 co-administered with lopinavir/ritonavir. Tocilizumab It is an IL-6 inhibitor. It increases expression of CYP3A4; however, no anticoagulant dose adjustments are currently recommended with concomitant use of tocilizumab at this time. There are no major drug-drug interactions between investigational COVID-19 therapies and parenteral anticoagulants. Considerations for venous thromboembolic (VTE) prophylaxis The World Health Organization interim guidance statement recommends prophylactic daily low-molecular weight heparins (LMWHs), or twice daily subcutaneous unfractionated heparin (UFH). If pharmacological prophylaxis is contraindicated, mechanical VTE prophylaxis (intermittent pneumatic compression) should be considered in immobilized patients. Every effort should be made to ensure that patients receive all scheduled doses of pharmacologic VTE prophylaxis because missed doses are associated with worse outcomes. In addition, once daily dosing regimen of LMWHs may be advantageous over UFH to reduce personal protective equipment (PPE) use and exposure of healthcare workers. Extended (post-discharge) VTE prophylaxis. After hospital discharge from acute medical illness, extended prophylaxis with LMWH or direct oral anticoagulants (DOACs) can reduce the risk of VTE, at the cost of increase in bleeding events. The consideration of extended prophylaxis (for up to 45 days) should be reasonable for patients with elevated risk of VTE (e.g., reduced mobility, co-morbidities such as active cancer, elevated D-dimer >2 times the upper normal limit) who have low risk of bleeding COVID-19 and interventional therapies for VTE Pulmonary embolism response teams (PERTs) allow for multidisciplinary care for patients intermediate and high-risk with VTE. During the COVID-19 pandemic, PERTs should transition from in-person inpatient evaluation to e-consults using phone calls or telemedicine systems whenever feasible. It is important to note that, there are minimal available data demonstrating lower mortality from routine use of advanced VTE therapies. Therefore, the use of catheter-directed therapies should be limited to the most critical situations. Recurrent PE despite optimal anticoagulation, or clinically-significant VTE in the setting of absolute contraindications to anticoagulation are among the few scenarios in which placement of an inferior vena cava filter may be considered. Intermediate-risk hemodynamically stable patients (intermediate-low risk, or intermediate-high risk PE according to ESC classification, sub-massive PE according to prior classifications) should be managed initially with anticoagulation and close monitoring. In case of deterioration, rescue systemic fibrinolysis should be considered, with catheter-directed options as an alternative. For patients with overt hemodynamic instability (high-risk PE according to the ESC classification, massive PE according to prior classifications) systemic fibrinolysis is indicated, with catheter-based therapies reserved for scenarios that are not suitable for systemic fibrinolysis. In cases of shock, if infection control settings are equal, bedside initiation of extracorporeal membrane oxygenation (ECMO) is preferred in cases with known COVID-19 positivity or uncertain status, rather than support strategies requiring the use of a catheterization laboratory or an operating room in order to limit unnecessary exposure. The vast majority of patients with symptomatic acute deep venous thrombosis (DVT), should be managed with anticoagulation, with home treatment whenever possible. The few that may require acute endovascular techniques (either local fibrinolysis or embolectomy) include those with phlegmasia, or truly refractory symptoms. |
| Management of ACS |
| Last updated: 05-10-21 |
Patients with STEMI admitted via the Accident and Emergency Department and in whom PPCI was performed
Inpatient STEMI (n=1), STEMI with unknown symptom onset time (n=3), and cardiac arrest patients (n=2)
Comparison Group: 108 patients with STEMI treated with PPCI in the prior year from February 1, 2018, to January 31, 2019 (N=108)
• Reduction in STEMI Cath lab activation in the USA during COVD-19. (Garcia S et al,JACC, 2020)
March 1 st was identified as the beginning of the “After COVID period (AC)”. In this paper they compared cath lab activation In 9 high volume centers (>100 PPCI/year) for STEMI before and after March 1 st. Study duration: Between Jan 1 st 2019 and March 31 st 2020.
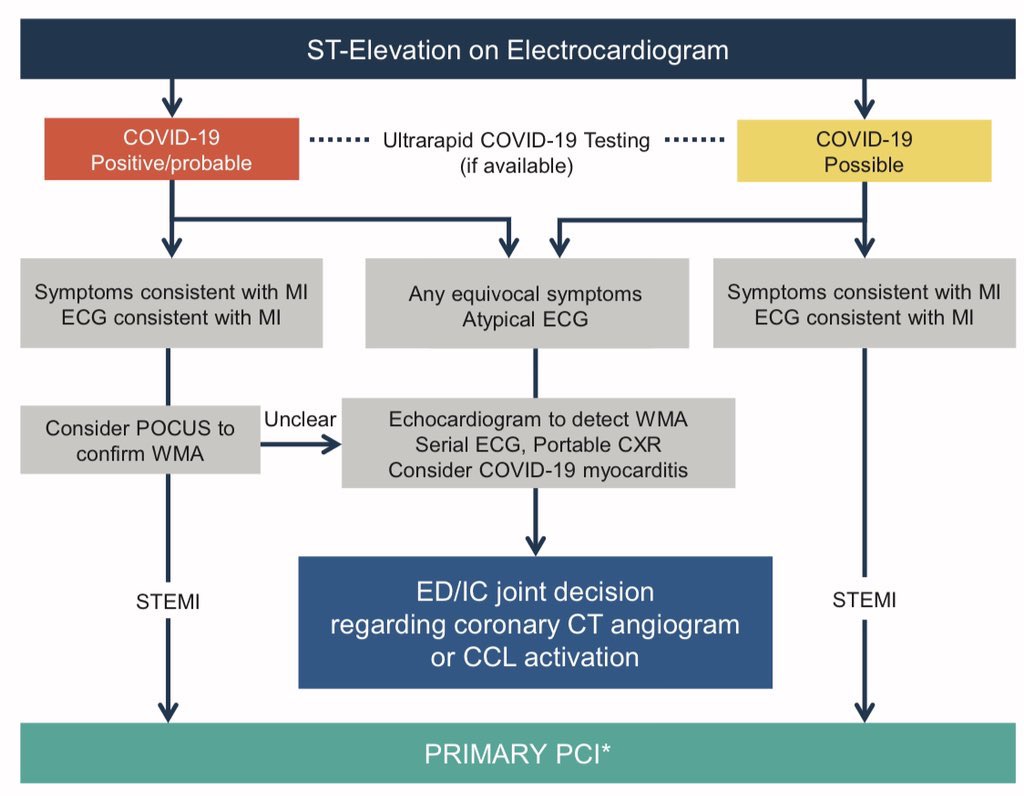
•38% decrease in STEMI activation (95% CI: 26,49; p<0.001) •All sites combined reported >180 STEMI activation every month (mean of 23.6 act/mo) in BC period. •All sites combined reported 138 activations/ month (mean 15.3 act/mo) in AC period. •These findings are similar to 40% reduction noticed in Spain. •The Spanish Society of Cardiology has reported a drop of 40%. (Rodriguez-Leor et al,Rec: Interventional Cardiology, 2020) • ST-Segment Elevation in Patients with Covid-19 — A Case Series. (Bangalore et al ,NEJM, 2020) •Patients with confirmed Covid-19 who had ST-segment elevation on electrocardiography were included in the study from six New York hospitals. •They identified 18 patients with Covid-19 who had ST-segment elevation •Median age of the patients was 63 years, 83% were men, and 33% had chest pain.total of 10 patients (56%) had ST-segment elevation at the time of presentation, and the other 8 patients had development of ST-segment elevation during hospitalization. •9 patients (50%) underwent coronary angiography; 6 of these patients (67%) had obstructive disease, and 5 (56%) underwent percutaneous coronary intervention (1 after the administration of fibrinolytic agents). •Total of 13 patients (72%) died in the hospital (4 patients with myocardial infarction and 9 with noncoronary myocardial injury). •Management of Acute Myocardial Infarction During the COVID-19 Pandemic (Mahmud et Al, JACC, 2020)
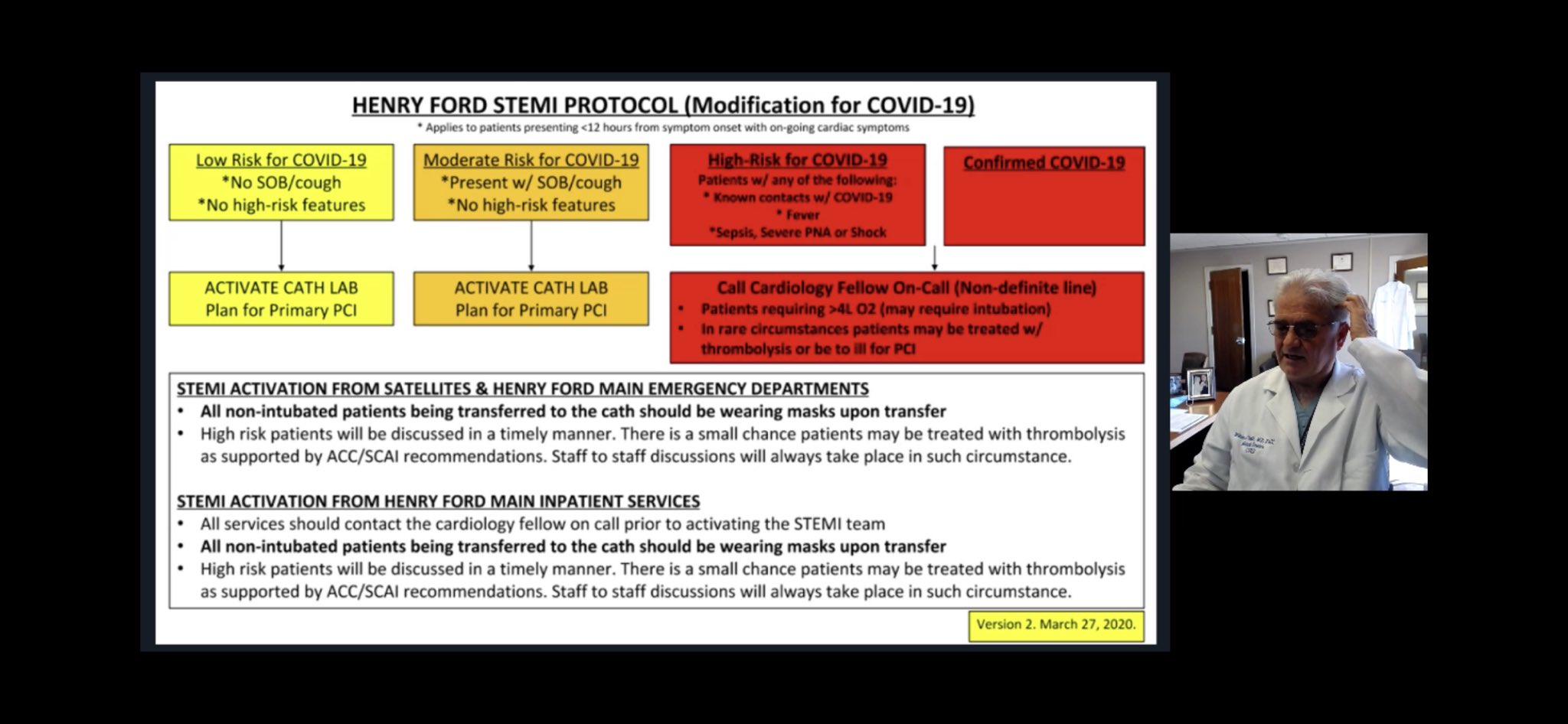
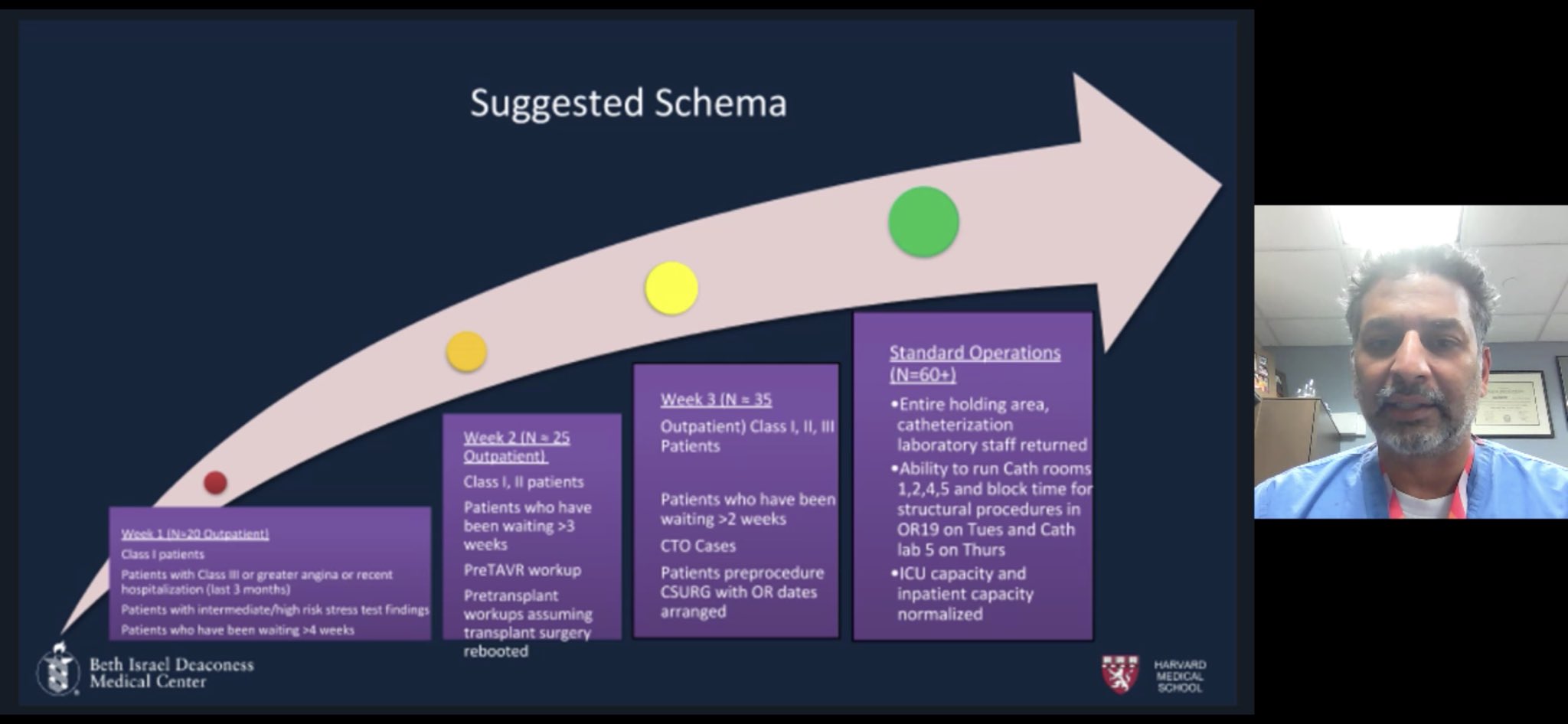
•A reasonable algorithm and timely discussion on STEMI management (Daniels et al, Circulation, 2020) •Institutional Protocols on ACS Management: Henry Ford STEMI Protocol Beth Israel Deaconess Cath Lab Protocol |
| Acute Cardiac Injury (ACI) |
| Last updated: 05-10-21 |
Presents with a broad spectrum and severity of symptoms
|
| Cardiogenic Shock and Mechanical Circulatory Support |
| Last updated: 05-10-21 |
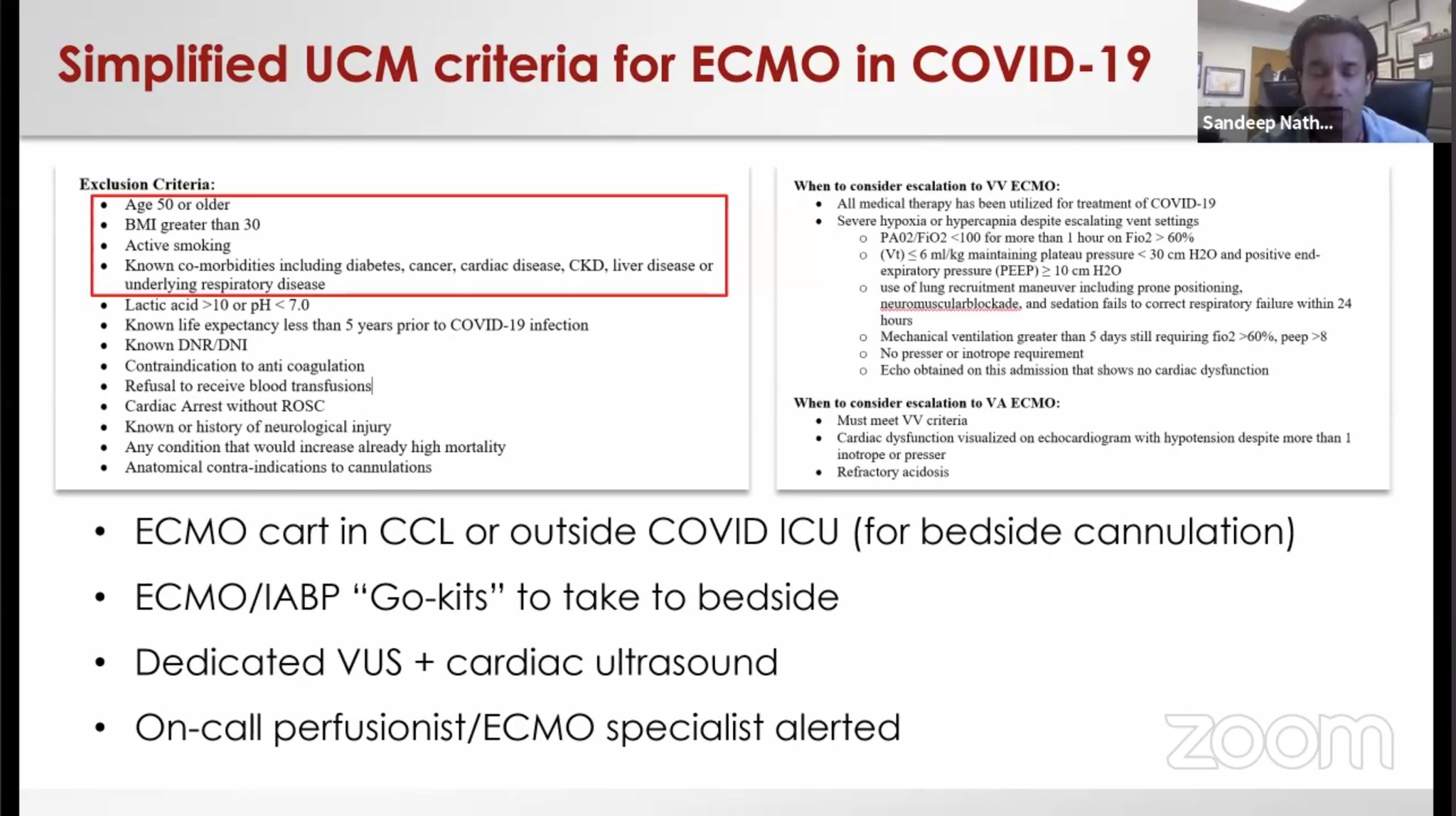
1.Cardiogenic Shock and Mechanical Support (Henry, Lancet , 2020) · Immunological status of patients should be considered when selecting candidates for ECMO · Elevated IL-6 concentrations in lung noted to induced by initiation of ECMO · Clinicians should consider tracking both lymphocyte count and IL-6 during ECMO to onitor patient status and prognosis · Patients who died from COVID-19 are reported to have had significantly lower lymphocyte counts than survivors 2. Cardiac Arrest: Cause of Death: (Arentz et al, JAMA 2020) Heart failure with respiratory failure (33%) Myocardial damage (7%) Outcome: Outcomes for in-hospital cardiac arrest with attempted resuscitation from China (Shao et al, Resuscitation, 2020)
Lombardia Cardiac Arrest Registry ( Baldi et al, NEJM, 2O2O) · Out of Hospital Card Arrest During Covid-19 in Italy · Increase of 58% relative to comparable period in 2019 · 77% of Outside Hospital Cardiac Arrests + · Highlights the importance of using all-cause mortality as an indicator of COVID-19 burden
Role of ECMO as described in the webinar by C3 Academy on the North American Covid Experience |
| PAD |
| Last updated: 05-10-21 |
|
| Structural Interventions |
| Last updated: 05-10-21 |
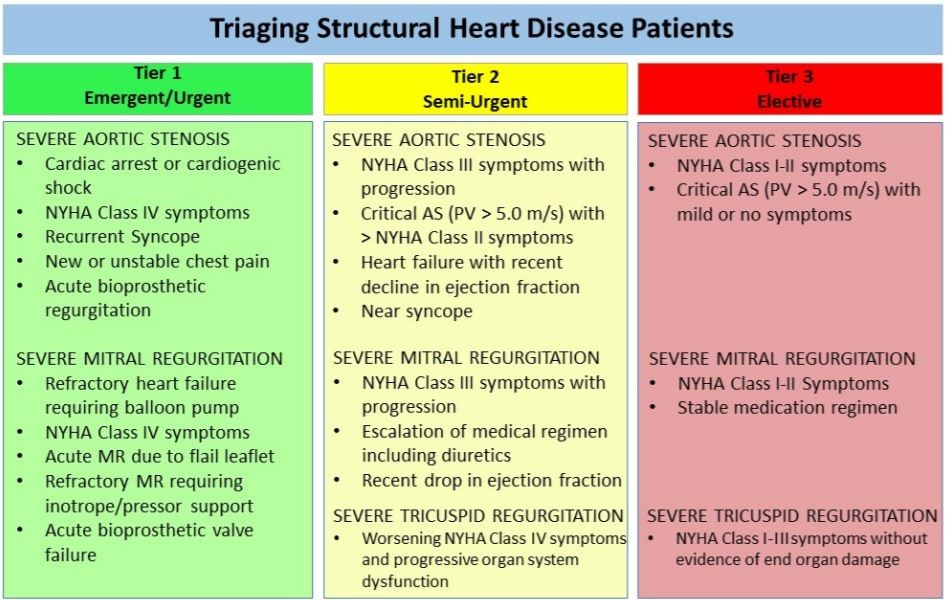
Triage Considerations for Patients Referred for Structural Heart Disease Intervention During the Coronavirus Disease 2019 (COVID‐19) Pandemic: An ACC/SCAI Consensus Statement (April 6). (Shah et al, CCI, 2020)
Intraprocedural Imaging Considerations: •Full PPE for the structural imager •Major aerosolization risk to the structural imager and cardiac anesthesia team during the initial intubation and any TEE probe manipulation thereafter in a non-intubated patient. •In already ventilated patient, HEPA filter be placed with the ET tube to maximize safety . Outpatient Clinic: •STS/ACC TVT registry allowed for substitution of in-person visits with telephone or virtual visits for the 30-day and 1-year follow-up visits.
|
| Thromboembolic Disease |
| Last updated: 05-10-21 |
Articles & Webinars discussing prevalence of coagulopathy and thromboembolic disease in COVID-19 patients: •Feature | Thrombosis and COVID-19: FAQs For Current Practice (Barnes et al,ACC Apr 22, 2020) •JACC Paper Outlines Implications, Considerations For Thrombotic Disease Patients During COVID-19 Pandemic (Bikdeli et al, JACC, 2020)
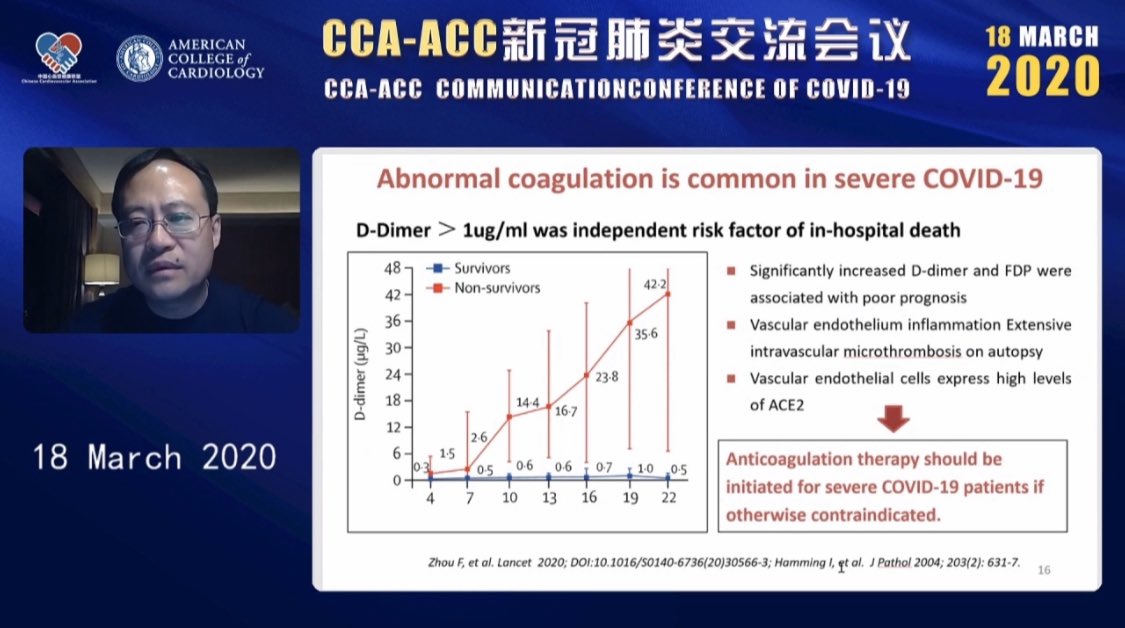
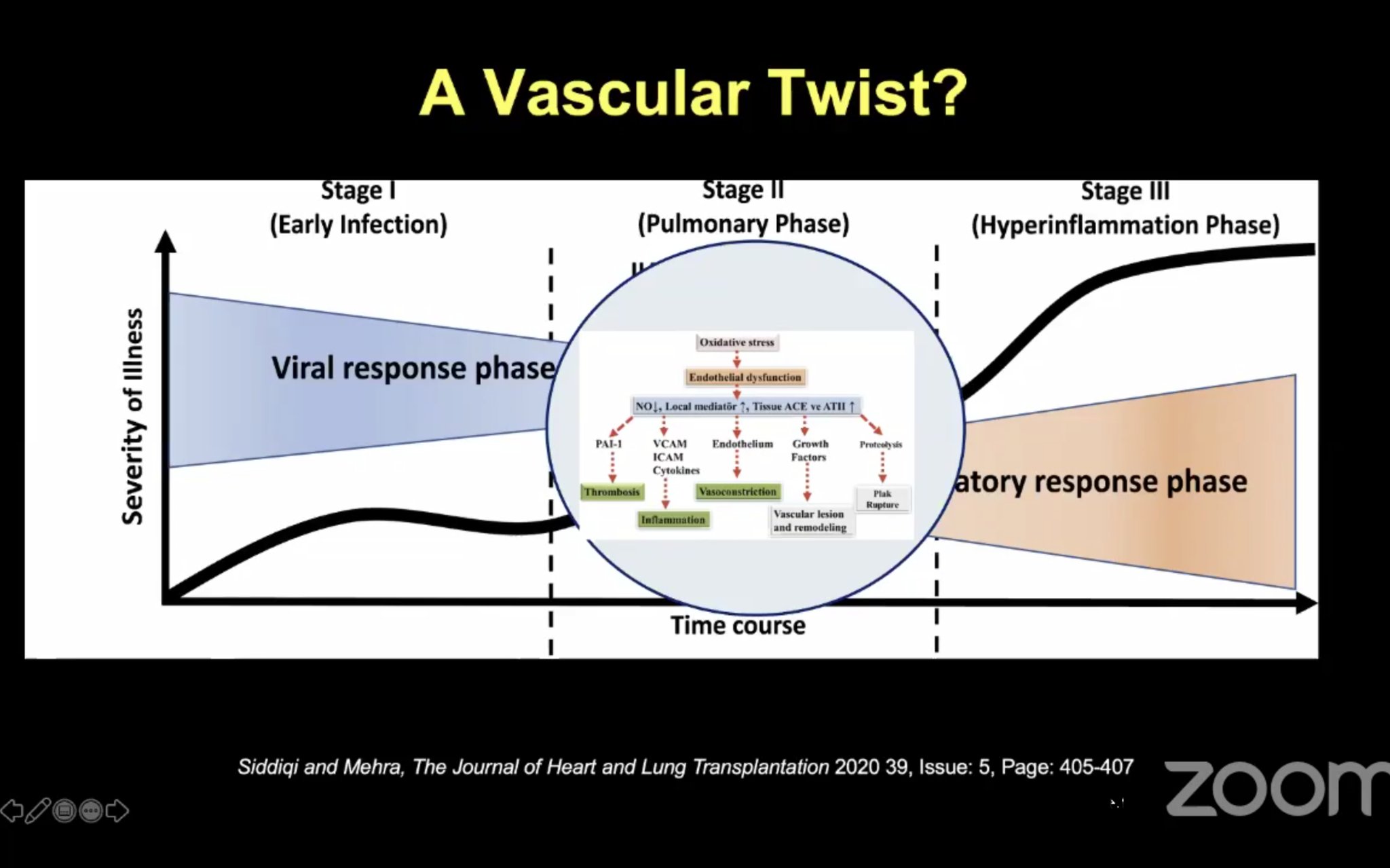
•Case of Venous Thromboembolism by Dr Rebecca Hahn MD (@hahn_rt ) •Thrombosis, Thromboprophylaxis & Coagulopathy in COVID- 19 Infections ISTH Webinar Link •ACC Webinar-the China Experience describing abnormal coagulation in COVID-19 (ACC Webinar, March 18, 2020) •C3 Academy Webinar Slide describing the inflammatory response: |
| Personal Protective Equipment |
| Last updated: 05-10-21 |
CDC guidelines Recommend N95 respirator, face shield, gown and gloves used by all code responders during code events (CDC Guidelines, 2020) Donning and Doffing Sequence: · Wear appropriate PPE during all procedures including N95 mask or powered air-purifying respirator (PAPR) before patient arrival. · Avoid compacting contaminated clothing in the waste container to prevent aerosolization. · Final mask removal takes place in the anteroom to avoid air droplet exposure. Equipment · Designate single procedure room · Use HEPA filters as an additional strategy for safe containment and elimination · Dedicated COVID-19 equipment cart · Minimize number of personnel Patient Transportation and Cleaning · Frequent wipe downs · Terminal clean · Entry into the room should be delayed after procedure
SCAI : Video link Another Link :courtesy Dr Bangalore MD C3 Academy Webinar explaining facility modification for PCI : Cathlab recommendations: reverse airflow, biplane suite, pre-packed equipment for dirty lab |
| Post Covid |
| Last updated: 05-10-21 |
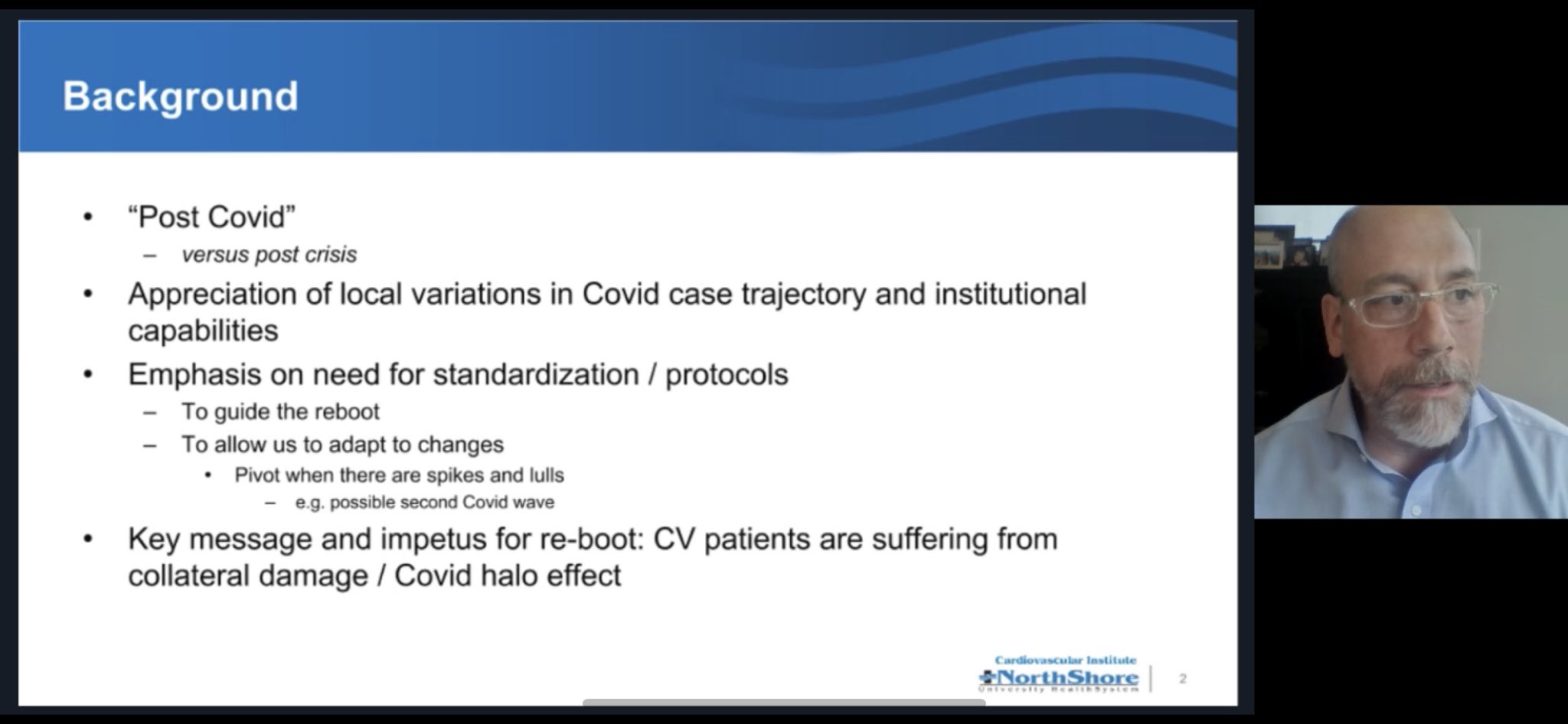
Webinar slide on post COVID-19 guidance from Northshore Hospital Safe Reintroduction of Cardiovascular Procedures and Diagnostic Tests during the COVID-19 Pandemic: Guidance from North American Societies (Wood et al, JACC, 2020) As regions move along the journey of managing the COVID-19 pandemic, there is an opportunity to reintroduce regular cardiovascular care in a progressive manner with appropriate safeguards. |
| Other Resources |
| Last updated: 05-10-21 |
|